On July 30th (Tuesday), the Supreme Court of India directed the Uttarakhand state government to make a decision regarding the suspension of 14 products manufactured by Patanjali Ayurved, a company founded by yoga guru Ramdev. The directive came from a bench of Justices Hima Kohli and Sandeep Mehta. The state government, led by the Bharatiya Janata Party (BJP), has been given a deadline of two weeks to address this issue.

Details of the Suspension
The suspension of these products stems from allegations that Patanjali Ayurved violated the 1945 Drugs and Cosmetics Rules. On April 29, the Uttarakhand State Licensing Authority for Ayurvedic and Unani Services reported to the court that licenses for Patanjali Ayurved's products had been suspended due to repeated breaches of regulatory standards.
The specific products affected by this suspension include:
-
Swasari Gold
-
Swasari Vati
-
Bronchom
-
Swasari Pravahi
-
Swasari Avaleh
-
Mukta Vati Extra Power
-
Lipidom
-
BP Grit
-
Madhugrit
-
Madhunashini Vati Extra Power
-
Livamrit Advance
-
Livogrit
-
Eyegrit Gold
-
Patanjali Drishti Eye Drop

Recent Developments
During a court hearing on July 9, Patanjali Ayurved informed the bench that it had directed its 5,606 franchise stores to withdraw these suspended products from sale. Additionally, the company stated that it had instructed media platforms to cease advertising these products. The court required Patanjali Ayurved to file an affidavit within two weeks to confirm whether social media intermediaries had complied with the withdrawal of advertisements.
At the most recent hearing, the Indian Medical Association (IMA) brought to the court's attention that despite these measures, the 14 products were still available over the counter, although Patanjali had reportedly ceased manufacturing them.
In response, Patanjali Ayurved claimed that the Uttarakhand government had lifted the suspension order on July 1, following a review by a committee appointed to investigate the issue. The Supreme Court was considering a petition from the IMA accusing Patanjali Ayurved of conducting a “smear campaign” against modern medicine and the Covid-19 vaccination initiative.
Delhi High Court Orders Removal of Coronil Posts
-
Court’s Directive
On Monday, the Delhi High Court issued a directive to Ramdev, instructing him to remove social media posts promoting Patanjali’s Coronil as a cure for Covid-19. This decision followed a plea from various doctors' associations, which accused Ramdev of making “unsubstantiated claims” about Coronil’s efficacy.
Justice Anup Jairam Bhambhani ordered Ramdev to delete the tweets within three days. Should he fail to comply, the court has mandated that the social media platform X (formerly Twitter) must remove the posts.
-
Allegations and Licensing Concerns
The petitioners argued that Ramdev had falsely advertised Coronil as a cure for Covid-19, while its licensed use was only as an immunity booster. This misrepresentation has been a significant point of contention.
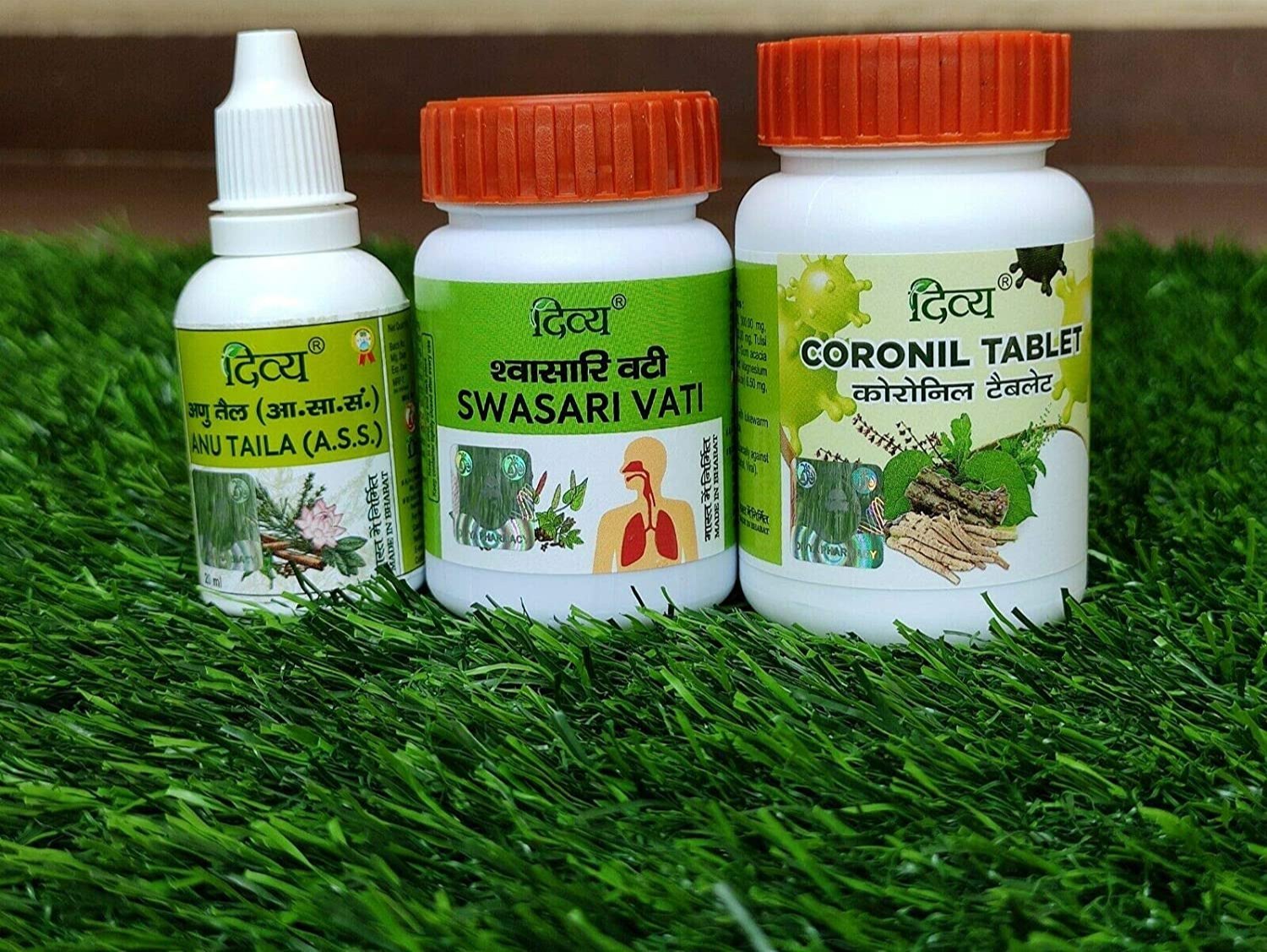
Bombay High Court Imposes Fine on Patanjali
- Violation of Court Orders
In a separate legal development, the Bombay High Court has imposed a fine of Rs 4 crore on Patanjali Ayurved. This fine was levied for violating a 2023 interim order that prohibited the sale of camphor products by the company.
Justice RI Chagla described Patanjali’s actions as a “wilful and deliberate breach” of the court’s order, indicating an intent to disregard legal directives. The court has instructed Patanjali Ayurved to deposit the fine within two weeks.
- Previous Penalties
Earlier, on July 8, the Bombay High Court had already ordered Patanjali Ayurved to pay Rs 50 lakh for breaching the injunction against the sale of camphor products. The additional fine reflects the court’s severe stance on the company’s non-compliance.
These legal actions underscore ongoing scrutiny and regulatory challenges faced by Patanjali Ayurved, impacting its operations and public statements.
With inputs from agencies
Image Source: Multiple agencies
© Copyright 2024. All Rights Reserved Powered by Vygr Media.

























