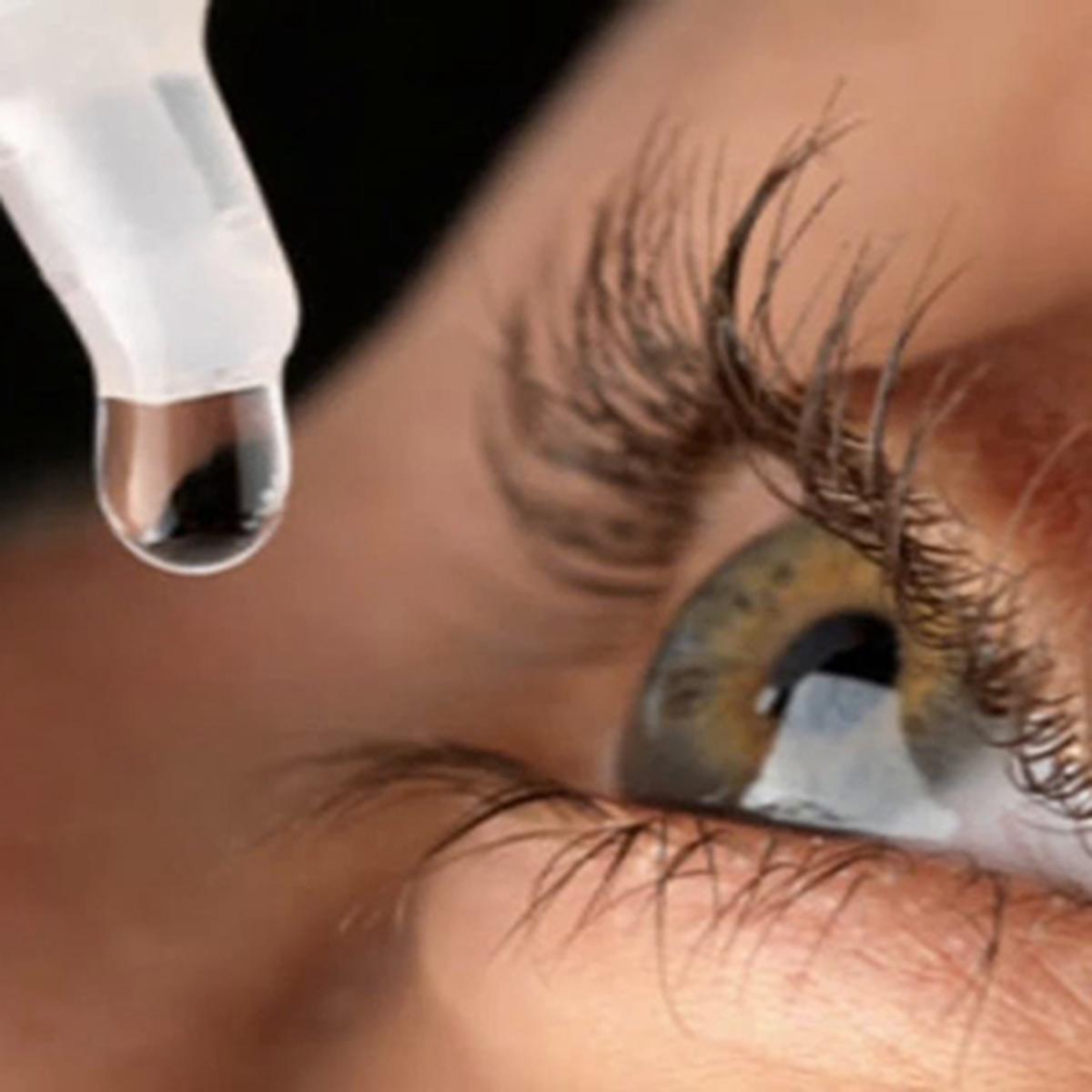
Three months have passed since the Sri Lankan government raised concerns over infections linked to an eye drop manufactured in Gujarat, India, affecting at least 50 patients. However, the Indian drug regulators have yet to respond to the situation, leaving Sri Lanka in limbo. Despite the lack of action from Indian authorities, Sri Lankan health officials have taken matters into their own hands, ceasing procurement of the eye drops from the manufacturer, Indiana Ophthalmics. The eye drops in question, containing methylprednisolone, was purchased by Sri Lanka in March from a Mumbai-based supplier. Tragically, in April, patients with cataracts who got the eye drop following their procedures in three hospitals in Colombo, Gampaha, and Nuwara-Eliya had serious infections.
Two individuals have even lost their eyesight due to the contaminated drops. Methylprednisolone eye drops are commonly used to reduce inflammation, redness, and itchiness after cataract surgeries. First sounding the alarm, Sri Lanka contacted the Gujarat-based producer, Mumbai-based supplier Alvita Pharma, and the Indian pharmaceuticals authority in May. But so far, no reply has been received. Professor SD Jayaratne, chairman of Sri Lanka's National Medicines Regulatory Authority (NMRA), expressed disappointment over the lack of communication between the Indian authorities and the manufacturer. In response to the outbreak of infections, Sri Lankan authorities asked Alvita Pharma to recall the contaminated eye drops.

Additionally, they informed the World Health Organization about laboratory reports confirming bacterial contamination in the eye drops. Indiana Ophthalmics, despite the warning from Sri Lanka, continues to export its products to more than 30 countries. This marks the third incident in the last six months involving ophthalmic products from Indian companies being flagged for poor quality. The United States previously issued an import alert against Global Pharma, and the World Health Organization warned against Galentic Pharma. Sri Lanka's experience highlights concerns about the quality standards of drugs from India and China, with the US Congress's Energy and Commerce Committee expressing worries about overreliance on drugs from these countries.
The Investigation by Sri Lanka
Upon receiving the methylprednisolone eye drops in March, Sri Lanka began experiencing infections among patients who had undergone cataract surgery. The first infection case was reported on April 4, with patients from the National Eye Hospital in Colombo and the District General Hospitals in Gampaha and Nuwara-Eliya being affected. The patients exhibited symptoms of eye infections caused by gram-negative oxidase-positive bacteria, a type of pathogen that can lead to vision loss if it causes severe eye infections. Such bacteria can spread through non-sterile surgical instruments or contaminated eye drops, making sterile products and careful procedures during surgery essential. Sri Lanka's National Medicines Regulatory Authority acted promptly, conducting tests on operation theatre instruments and eye drops. The lab reports confirmed bacterial contamination in both the patients and the eye drops, prompting the withdrawal of all methylprednisolone eye drops manufactured by Indiana Ophthalmics. Fortunately, no new infection cases emerged after the withdrawal, but two patients treated with the contaminated eye drops lost their vision. Several others had to be treated after being referred from other hospitals.
Indian Regulators Stay Silent
While Sri Lanka took action and raised the issue with Indian regulators, the response from Indian authorities has been lacking. The Gujarat Food and Drug Administration inspected Indiana Ophthalmics' Wadhwan plant but found no major deviations in their manufacturing practices. However, the Central Drugs Standard Control Organisation is yet to communicate the lab reports regarding the eye drops. The Pharmaceuticals Export Promotion Council of India also intervened by requesting product details and licenses from Indiana Ophthalmics, but they did not suspend the company's membership. The final decision and necessary action lie with the CDSCO based on their investigation. The investigation in Sri Lanka has left no doubt that the eye drops were responsible for the infections, while Indian regulators claim there have been no prior complaints about the manufacturer. Alvita Pharma, the supplier, did not respond to inquiries regarding the quantity of recalled eye drops. As the authorities continue to grapple with the situation, concerns about drug quality standards persist, demanding a more proactive and collaborative approach from drug regulators to ensure patient safety.
© Copyright 2023. All Rights Reserved Powered by Vygr Media.