Elon Musk's brain implant startup Neuralink has received approval from an independent review board to begin recruiting participants for its first human clinical trial. The company is actively recruiting people with paralysis to participate in a comprehensive six-year study and test its experimental equipment.
Neuralink is part of a group of companies working on Brain-Computer Interfaces (BCIs) designed to capture and interpret brain signals. But Elon Musk's much-hyped efforts (including efforts to develop comprehensive brain-computer interfaces to keep pace with artificial intelligence) have sparked skepticism and ethical concern among neuroscientists and experts. 
Last year, the Food and Drug Administration (FDA) initially denied Neuralink's request to accelerate human trials. However, in May, the FDA granted Neuralink an Investigational Device Exemption (IDE), citing several concerns that needed to be addressed before approving human trials. These concerns focused on the lithium battery, the potential for implant wire migration within the brain, and how to safely remove the device without damaging brain tissue. The FDA has not said how it addressed those initial concerns.
Neuralink is actively seeking patients with quadriplegia due to vertical spinal cord injury (ALS). These participants will receive surgical implantation of a BCI into the brain area responsible for movement using a unique robot. The ultimate goal is to allow individuals to control a computer cursor or keyboard using only their thoughts. The research focus is primarily on evaluating the safety and functionality of the technology.
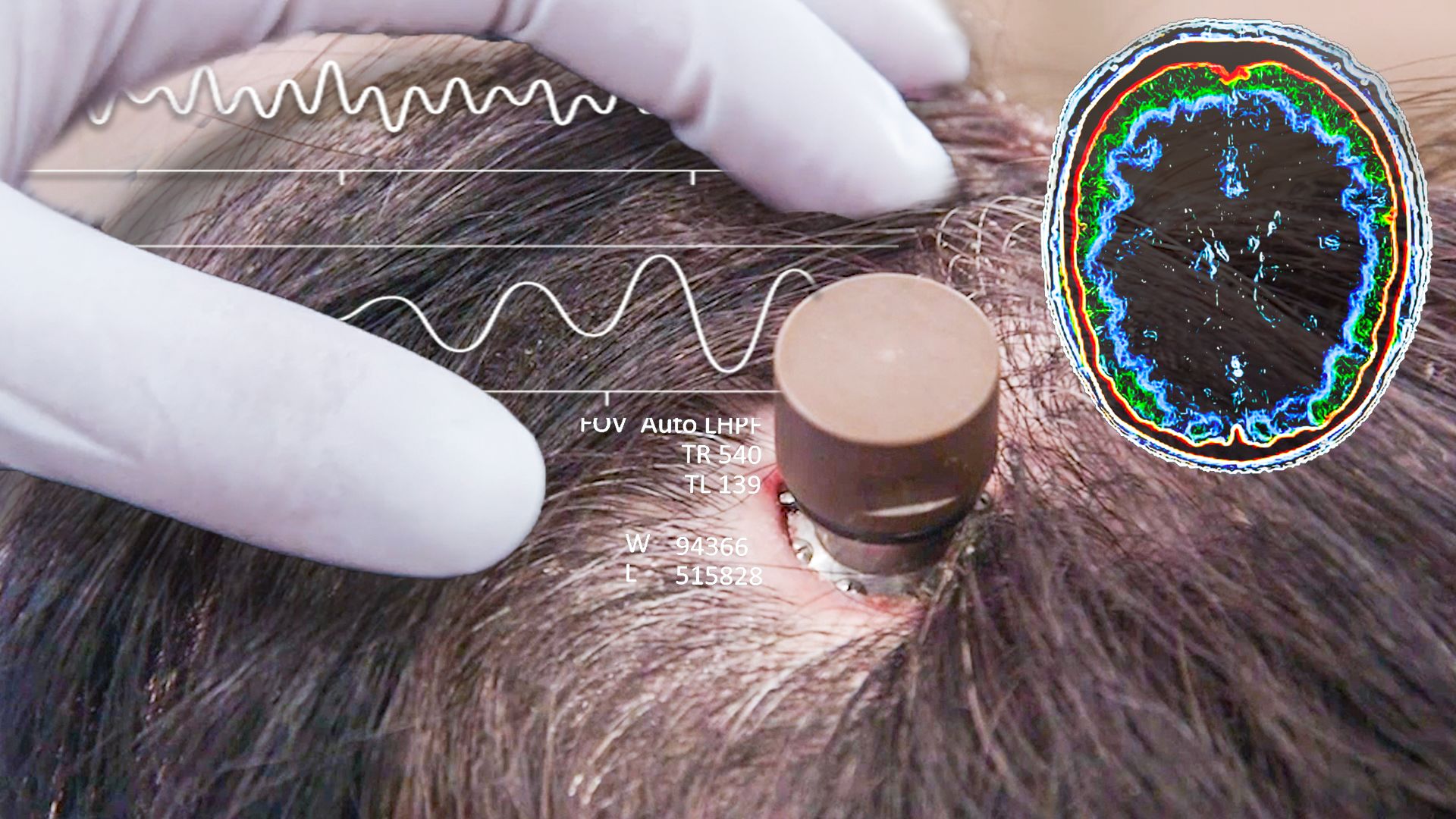
This announcement from Neuralink follows prior regulatory approval for the study. However, their previous animal experiments have come under intense scrutiny due to reports that they caused unnecessary suffering. Former employees said these tests were inadequate and that in some cases, improperly fitting devices to pigs led to euthanasia. These allegations have sparked several investigations, including by the Department of Agriculture, Animal Welfare, and Department of Transportation into improper handling of biohazardous materials across state lines.
Neuralink has not yet provided specific information about the study's time and location, number of participants, etc. If their device proves safe for human use, it could take years to pass through the testing phase and become available to patients.
Elon Musk has long shared his ambitious vision for Neuralink, envisioning a future where both disabled and able-bodied people can easily receive surgical implants at local facilities. These implants are intended to treat a wide range of conditions, from obesity, autism, depression, and schizophrenia to activating functions such as internet surfing and telepathy.
© Copyright 2023. All Rights Reserved Powered by Vygr Media.