The government-run national health system in Britain will administer a cancer-treating injection to hundreds of patients in England for the first time. This injection could cut the recovery period in half.
Following authorization from the Medicines and Healthcare Products Regulatory Agency (MHRA), NHS England announced on Tuesday that hundreds of eligible patients receiving the immunotherapy atezolizumab were scheduled to receive "under the skin" injections. This will allow cancer teams to devote more time to treating patients.
"This approval will not only allow us to deliver convenient and faster care for our patients but will enable our teams to treat more patients throughout the day," Dr Alexander Martin, a consultant oncologist at West Suffolk NHS Foundation Trust said.
Atezolizumab, also known as Tecentriq, is typically administered to patients intravenously, straight into their veins, via a drip. This could frequently take up to an hour for certain patients because it can be challenging to find a vein, according to NHS England.
"It takes approximately seven minutes, compared with 30 to 60 minutes for the current method of an intravenous infusion," Marius Scholtz, Medical Director at Roche Products Limited said.
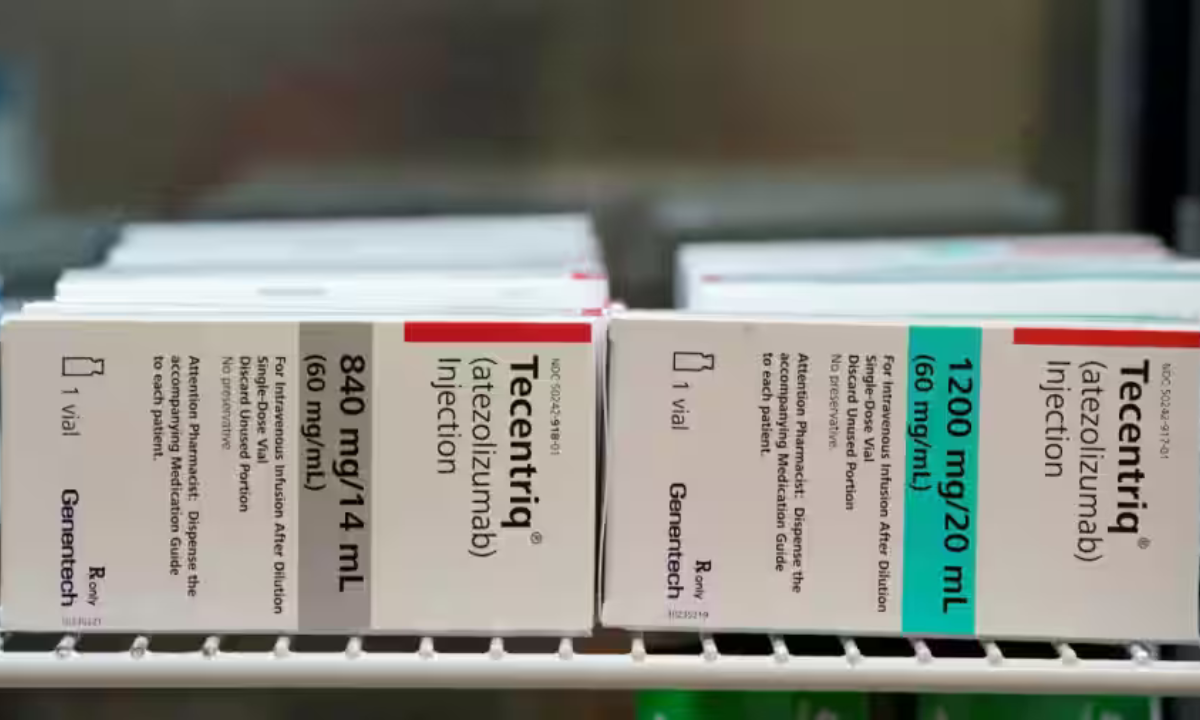
Atezolizumab, a Roche-manufactured immunotherapy medication, activates the body's own immune system to find and eliminate malignant cells. Currently, individuals on the NHS who have a variety of malignancies, such as lung, breast, liver, and bladder, can receive the treatment through transfusion.
According to NHS England, the majority of the roughly 3,600 people in England who begin atezolizumab treatment each year will switch to the quicker injection.
However, it was also mentioned that patients getting atezolizumab along with intravenous chemotherapy can continue receiving transfusions.
© Copyright 2023. All Rights Reserved Powered by Vygr Media.



















