The World Health Organization (WHO) has strongly criticised India's pharmaceutical sector after a deadly batch of cough syrup was linked to the deaths of at least 21 children in Madhya Pradesh. The children, all under five years old, reportedly died over the past month after consuming the medicine, which tests revealed was contaminated with a toxic industrial solvent. This alarming discovery has triggered immediate calls for better oversight and stricter safety protocols in India's massive pharmaceutical industry, casting a global spotlight on the country's drug manufacturing standards.
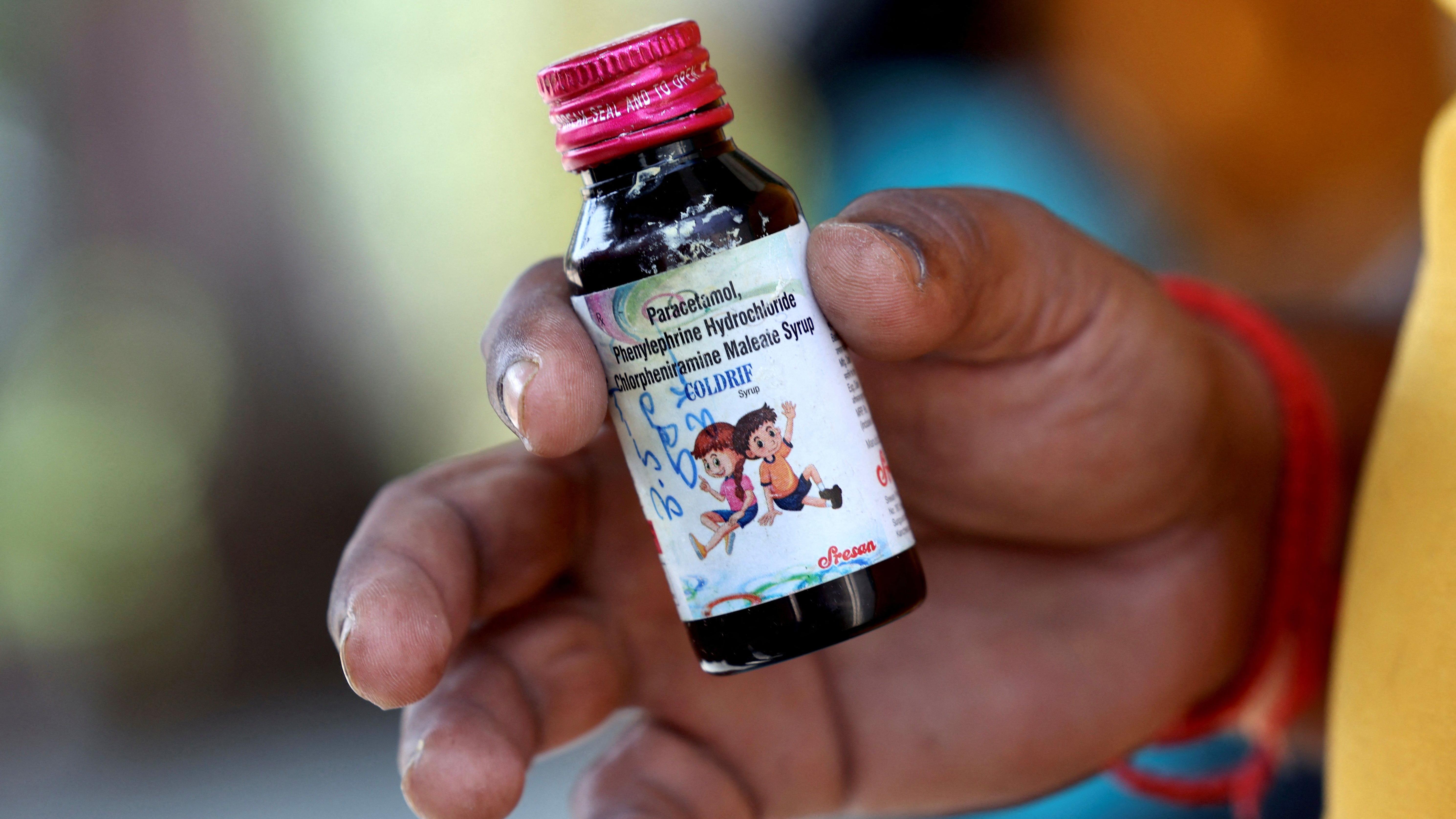
In Madhya Pradesh—a state in central India—doctors prescribed a cough syrup called Coldrif, produced by Sresan Pharma, to young children with common colds or coughs. Instead of healing the children, the medicine led to severe poisoning and tragic loss of life. Investigations found the syrup was contaminated with diethylene glycol (DEG), a substance commonly used in industrial solvents and antifreeze. Even tiny amounts of DEG are deadly when consumed, causing kidney failure, brain damage, and death. The government responded swiftly by banning the product in several states, whilst police arrested the owner of Sresan Pharma, G. Ranganathan, who now faces serious charges, including culpable homicide and drug adulteration.
The World Health Organization reacted sharply to these developments. Its officials have asked India for clarification regarding whether the toxic syrup was also exported to other countries—a pressing concern, given India's position as the world's third-largest producer of pharmaceuticals by volume, after the United States and China. India supplies over 50% of the world's generic medicines and vaccines, making any safety concerns a global health issue. The WHO's criticism comes amid growing international scrutiny of Indian pharmaceutical exports, particularly following similar incidents in recent years.
This is not an isolated event, and that's what makes it particularly troubling for global health authorities. In recent years, Indian cough syrups have faced international scrutiny due to similar tragedies across multiple continents. More than 70 deaths occurred in Gambia in 2022 after children consumed syrup imported from India, whilst in Uzbekistan, 68 children died between 2022 and 2023 in similar circumstances. The U.S. FDA and WHO have investigated multiple cases across Asia and Africa where contaminated syrups were involved in over 300 child deaths. These repeated incidents have created a pattern that international health agencies can no longer ignore, leading to increased pressure on Indian regulatory authorities.

The question many are asking is why these incidents keep happening in one of the world's largest pharmaceutical manufacturing hubs. While India's pharmaceutical industry powers a significant portion of the world's supply of affordable medications, its regulatory systems sometimes fail to keep pace with rapid growth and increasing demand. Weak quality control, inconsistent oversight, and lapses in safety checks can result in dangerous products reaching shelves and, tragically, vulnerable patients. The sheer scale of India's pharmaceutical sector—with thousands of manufacturing units spread across the country—makes comprehensive monitoring challenging. Many smaller manufacturers operate with minimal oversight, creating gaps where contaminated products can slip through the system.
The recent incident has intensified international scrutiny, with global health agencies urging India to enforce higher manufacturing standards and introduce tougher checks to guarantee drug safety before medicines enter the market or are exported abroad. The European Medicines Agency and the U.S. FDA have both increased inspections of Indian pharmaceutical facilities, whilst some countries are considering additional testing requirements for Indian-made medicines. This heightened scrutiny comes at a time when India is trying to position itself as the "pharmacy of the world," making these safety concerns particularly damaging to the country's reputation.
India has earned global recognition for producing low-cost, life-saving drugs, making healthcare more accessible worldwide, particularly in developing nations that cannot afford expensive branded medicines. The country's pharmaceutical exports are worth over $25 billion annually, and millions of people depend on Indian-made medicines for their daily health needs. However, repeated cases of contaminated medicines risk eroding this trust. Countries relying on Indian pharmaceuticals may start demanding extra testing, implementing stricter import controls, or seeking drugs elsewhere, which could ultimately harm global access to affordable medicines.

On the other hand, these incidents shed critical light on a widespread challenge that extends beyond India's borders. Countries everywhere, not just India, can struggle to maintain rigorous drug standards amidst rising demand and cost pressures. Global experts highlight the need for regulatory systems that are transparent, accountable, and proactive rather than reactive. The pharmaceutical industry worldwide faces constant pressure to reduce costs whilst maintaining quality, and this balance becomes particularly challenging in developing countries where resources for oversight may be limited.
The immediate response to the Madhya Pradesh tragedy has been swift but raises questions about long-term solutions. State governments have banned Coldrif and similar products, whilst law enforcement has launched criminal investigations against manufacturers and company owners. The WHO, U.S. FDA, and other agencies are partnering with Indian authorities to trace the origins and supply chains of the contaminated syrups. Health ministries are increasing checks on pharmaceutical factories, both for domestic and export purposes, and India has begun discussions with the WHO to address safety standards, aiming to restore global confidence in its medicines.

The tragedy in Madhya Pradesh serves as a wake-up call—not just for India, but for the global pharmaceutical supply chain. It highlights the need for stronger international cooperation in drug safety, better tracking systems for pharmaceutical products, and more robust regulatory frameworks that can keep pace with the global nature of modern medicine manufacturing. Consumers should remain vigilant and ask questions about drug safety, but governments, both local and global, share the responsibility of ensuring medicines are safe before reaching those who need them, especially vulnerable populations like children. Only through coordinated efforts between manufacturers, regulators, and international health agencies can we ensure that every bottle of medicine helps rather than harms, preserving both lives and the trust that underpins global healthcare systems.
With inputs from agencies
Image Source: Multiple agencies
© Copyright 2025. All Rights Reserved. Powered by Vygr Media.

























