A new Dengue vaccine ‘TAK-003’ by Japanese Company, Takeda has been prequalified by the World Health Organization (WHO). This is the second vaccine after ‘Dengvaxia’ to be prequalified.
About the vaccine ‘‘TAK-003’
Developed by the Japanese multinational pharmaceutical company Takeda, TAK-003, is a live-attenuated vaccine designed to target all four serotypes of the dengue virus – DENV-1, DENV-2, DENV-3, and DENV-4.
In Simpler terms, the vaccine contains weakened forms of each serotype, which will enter the body after vaccination allowing the body to build immunity. It will not cause the disease. However it is not certain that Immunity to one serotype will fully protect against others as they have genetic differences.
WHO recommendations on the usage of TAK-003
-
Only in Children aged 6–16 years in settings with high dengue burden and transmission intensity.
-
The vaccine is administered in a two dose schedule with a three-month interval between doses.
In February 2024, it was announced that Takeda Pharma would join forces with Hyderabad-based Biological E to produce 50 million dengue vaccines. Company executives also mentioned at the time that they were working with Indian regulatory authorities to obtain licensure.
This was a crucial aspect of Takeda's strategy to increase vaccine supplies in India and worldwide, as stated by Gary Dubin, Takeda’s President of the Global Vaccine Business Unit.
The UK, Brazil, Argentina, Indonesia and Thailand have also approved TAK-OO3.
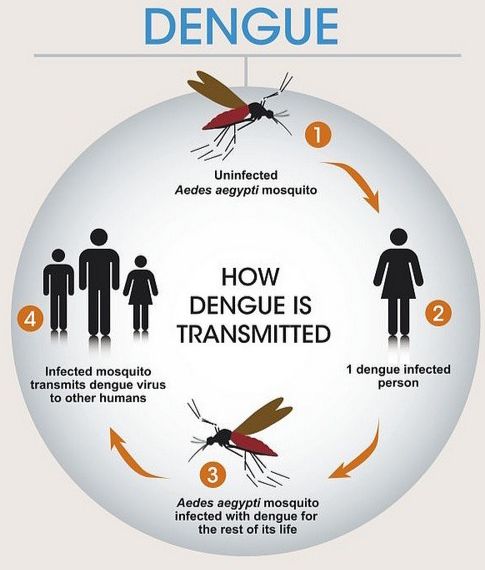
Why is Prequalification Important?
Dengue, a mosquito-borne viral infection, has been a major health threat to the world, particularly in tropical and subtropical regions.
With an estimated 100 to 400 million cases occurring worldwide annually, the disease exacts a heavy toll on affected communities.
Observing the threat, WHO prequalification of a second vaccine paves the way for easier adoption of the vaccine in countries, particularly in low- and middle-income countries where the burden of dengue is often most acute.

Moreover, prequalification of a vaccine also determines that the quality, safety, and efficacy has been assessed and approved according to the global standards, allowing for wider distribution and use in public health programs in areas that require it the most.
Dr Rogerio Gaspar, WHO Director for Regulation and Prequalification said, “The prequalification of TAK-003 is an important step in the expansion of global access to dengue vaccines, as it is now eligible for procurement by UN agencies including UNICEF and PAHO.'
“With only two dengue vaccines to date prequalified, we look forward to more vaccine developers coming forward for assessment so that we can ensure vaccines reach all communities who need it,” he added.
About the ‘First’ Vaccine- Dengvaxia
The first vaccine Dengvaxia ‘CYD-TDV’ against dengue, developed by Sanofi Pasteur, was prequalified in 2015. The vaccine Dengvaxia, known by its scientific name CYD-TDV, is a live attenuated tetravalent vaccine employed for preventing dengue fever in humans. It requires three separate injections, with the first dose followed by two more shots administered six and 12 months apart.
CYD-TDV is a vaccine made using genetic technology. It swaps certain genes from the yellow fever vaccine with genes from the dengue virus. This helps the body defend against all four types of dengue virus.

The vaccine has been approved in 19 countries and the European Union, but it is not approved in the US for use in individuals not previously infected by any dengue virus serotype or for whom this information is unknown.
The CYD-TDV vaccine has shown partial effectiveness in preventing dengue infection, particularly in those who have previously been infected with dengue.
However, it's associated with a risk of severe disease in individuals who haven't had prior dengue infection and then contract the disease after vaccination, so very limited in usage.
NATIONAL DENGUE DAY
May 16 is observed as a n National Dengue Day,the theme of 2024 is ‘Dengue Prevention: Our Responsibility for a safer tomorrow.’
it is crucial to take this communicable disease seriously because it has been endemic in over 100 countries. Dengue is thought to affect between 100 and 400 million people annually worldwide, with 3.8 billion of those cases occurring in dengue-endemic nations, primarily in Asia, Africa, and the Americas.
Symptoms of Dengue
-
High fever
-
headache
-
body aches
-
nausea
-
Fatigue and Restlessness
-
Rash
-
belly pain
-
vomiting
-
bleeding from the nose or gums,
-
extreme fatigue
-
Swollen glands
-
Feeling weak

Precautions against Dengue
-
Keep your surroundings clean
-
Do not let any still water collect near your localities.
-
Wear full length clothes
-
Apply Mosquito repellent cream on your body, use mosquito nets, or use killing spray.
-
Take special care in Monsoons, when the chances of Mosquito-borne diseases surges
Overall, prequalification of TAK-003 by the WHO is a major step forward in the global fight against dengue. We are getting closer to a time when the burden of dengue will be taken off. But for this, communities around the world needs to be prepared to face this serious public health issue by collective efforts, one of those is increase access to these vaccines.
Image Source: Multiple Agencies
Ⓒ Copyright 2024. All Rights Reserved Powered by Vygr Media.