The Central Drugs Standard Control Organisation (CDSCO), the country's top health regulatory body, has taken action to prohibit the use of a commonly used anti-cold medicine combination among children under four years old. Specifically, pharmaceutical companies producing GlaxoSmithKline's T-Minic Oral Drops, Glenmark's Ascoril Flu Syrup, IPCA Laboratories' Solvin Cold Syrup, and similar products have been directed by the regulator to include a 'warning.'

Directive and Concerns:
In a letter issued on December 18, the CDSCO instructed states and Union Territories to update the package inserts for products containing a combination of two medicines: chlorpheniramine maleate and phenylephrine. This combination is known for alleviating cold and flu symptoms like watery eyes, runny nose, sneezing, and throat or nasal itching. Chlorpheniramine maleate acts as an anti-allergic agent, while phenylephrine functions as a decongestant by narrowing blood vessels to ease nasal congestion.

Infant Safety Concerns:
Initially considered rational, this fixed-dose combination was under scrutiny and had previously received approval from a government committee chaired by Prof. Kokate. However, subsequent concerns arose regarding its use among infants and young children. The Subject Expert Committee (Pulmonary) recommended against its use in children below four years old due to safety concerns.
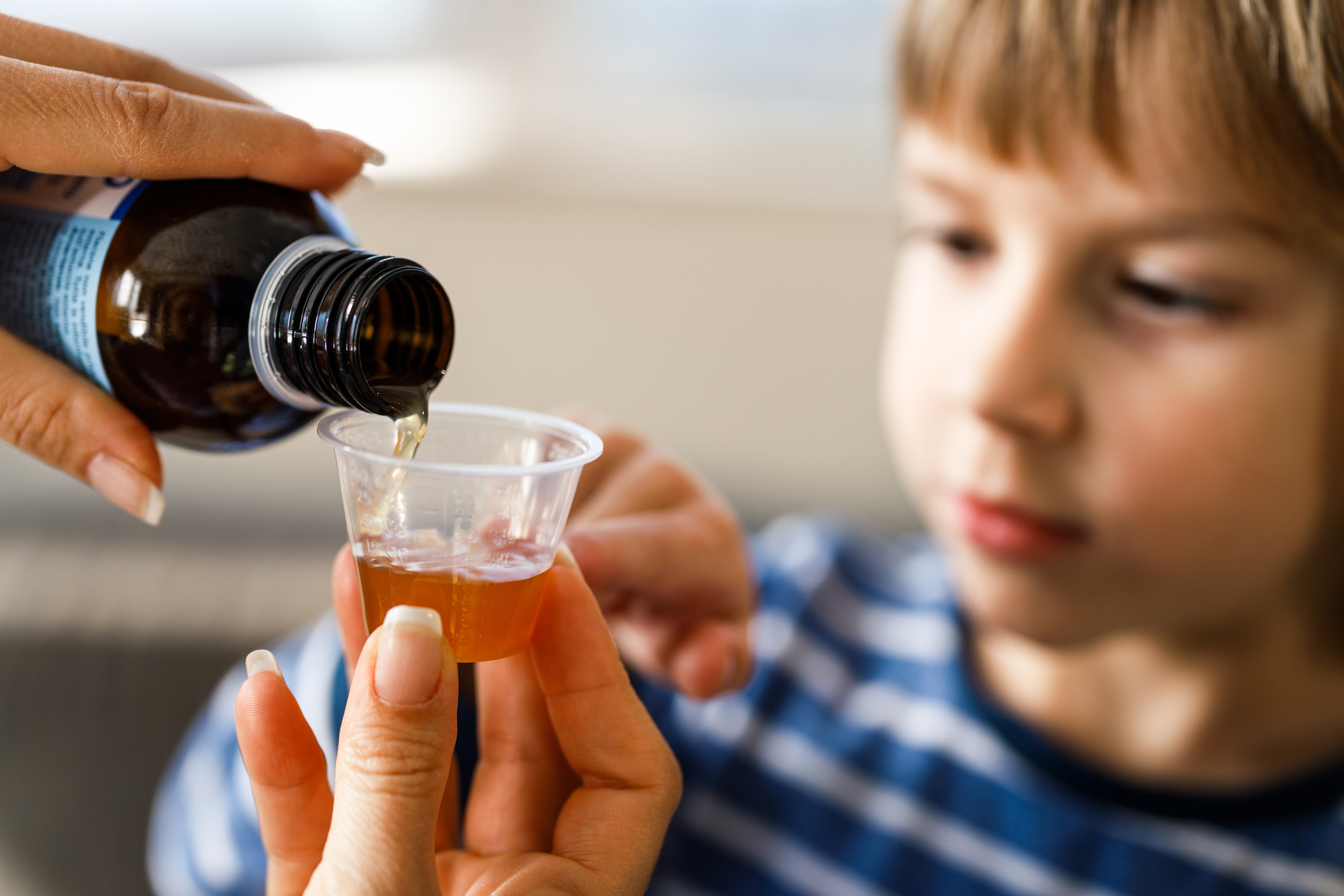
International Precedents and Expert Insights:
Experts point out that developed countries like the United States and the European Union had already prohibited similar products for infants and young children almost a decade ago. Paediatricians emphasize that these combinations lack evidence-based support for infants and could pose risks if used without supervision. Citing a U.S. study, one doctor noted that the U.S. regulatory agency had banned cough and cold medicines for children under six in 2007 due to potential side effects, with approximately 7,000 children annually requiring emergency treatment for related issues.

Awareness and Action:
Despite these warnings and regulatory actions, a significant number of caregivers in the United States remained unaware of the risks, potential side effects, and interactions with other medications associated with these products.
The decision by the CDSCO to caution against the use of these medications for children under four aligns with international precedents and safety concerns raised by experts regarding the potential risks these medicines pose to infants and young children.
© Copyright 2023. All Rights Reserved Powered by Vygr Media.