Baba Ramdev’s Patanjali Ayurved Ltd has halted the sale of 14 products after their manufacturing licenses were suspended by the Uttarakhand State Licensing Authority in April.

Licensing Suspension Prompts Patanjali Product Withdrawal:
Patanjali informed the Supreme Court on Tuesday that it has instructed its 5,606 franchise stores to withdraw these products, including
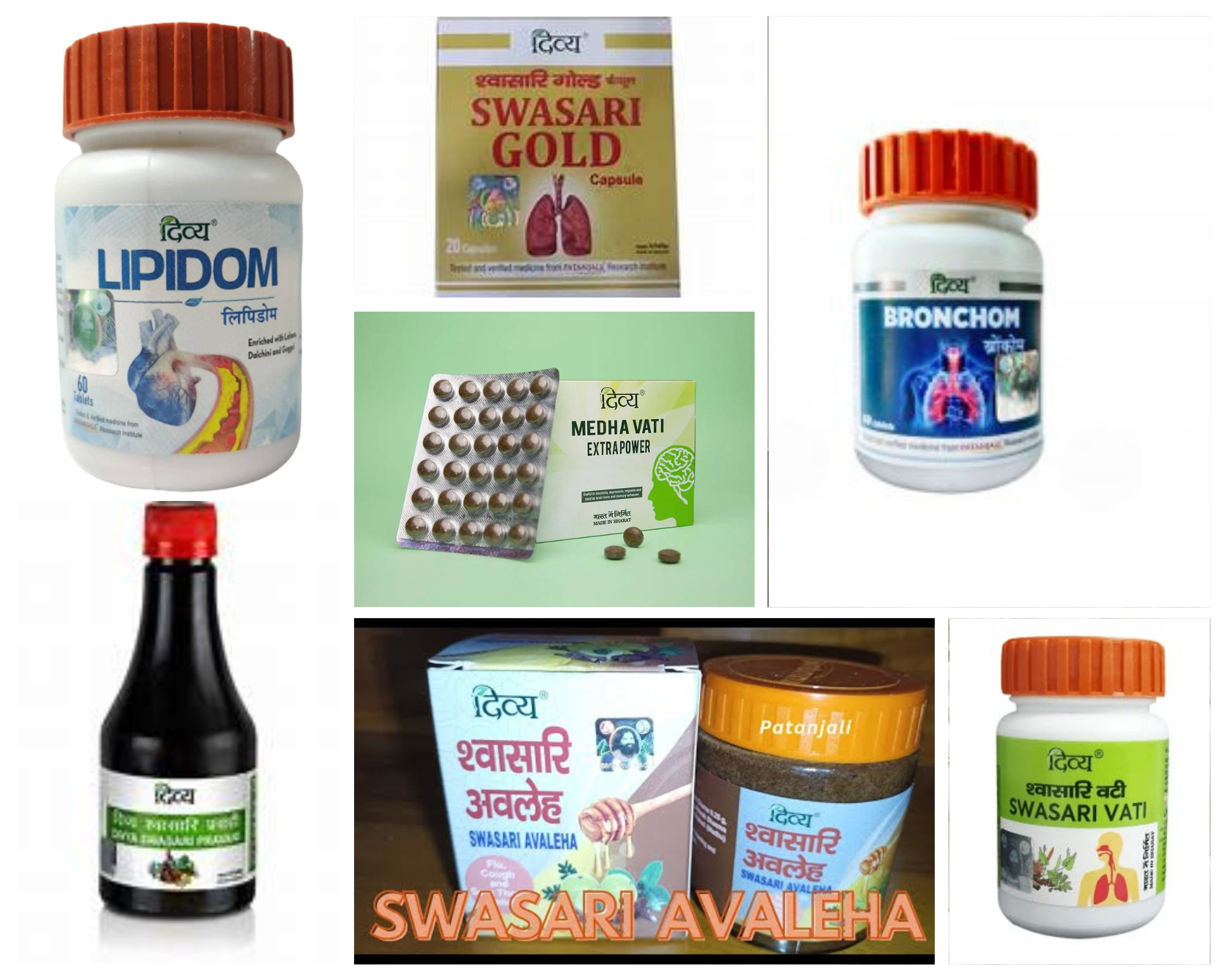
- Swasari Gold
- Swasari Vati
- Bronchom
- Swasari Pravahi
- Swasari Avaleh
- MuktaVati Extra Power
- Lipidom
- Bp Grit
- Madhugrit
- Madhunashini Vati Extra Power
- Patanjali Drishti Eye Drop
- Eyegrit Gold
- Livogrit
- Livamrit Advance.
Media Platforms Ordered to Remove Advertisements:
Patanjali also stated that media platforms have been directed to remove advertisements for these products. The company must file an affidavit within two weeks detailing whether social media intermediaries have complied with these requests and confirming the removal of all advertisements.
_1720534400.png)
A bench comprising Justices Hima Kohli and Sandeep Mehta has scheduled the next hearing for July 30.
This legal issue stems from a plea by the Indian Medical Association (IMA), accusing Patanjali of a smear campaign against the COVID-19 vaccination drive and modern medicine. The Supreme Court emphasized that products with suspended licenses should not be sold, with Justice Amanullah stating, "If the license is suspended, the product should not be sold."

Supreme Court Directive and Next Hearing:
The Uttarakhand State Licensing Authority had earlier informed the Supreme Court about the immediate suspension of manufacturing licenses for these 14 products. The court also questioned Patanjali about the continued presence of misleading advertisements online, demanding action to remove them. Further updates are expected at the next hearing.
With inputs from agencies
Image Source: Multiple agencies
© Copyright 2024. All Rights Reserved Powered by Vygr Media.

























